These 19th-century green books are beautiful but poisonous

|
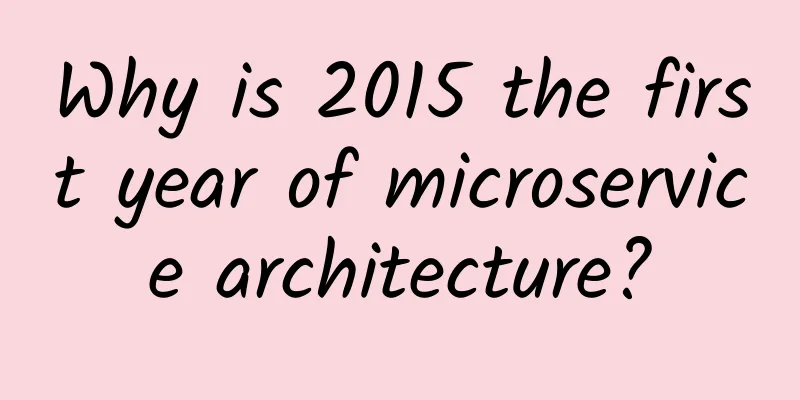
Image source: PhilaAthenaeum via Youtube The book will be damaged, but the toxicity will remain. In the spring of 2019, Dr. Melissa Tedone of the Winterthur Museum in Delaware, USA, borrowed an old book published in 1857 from the library. It is a home decoration guide called "Rustic adornments for homes of taste". The book is beautiful. Although it has been more than a century, its exterior still retains its bright green color, which is in stark contrast to the gold-plated fonts and patterns. As one of the collections to be exhibited at the Winterthur Art Museum, even though the spine and cover are about to fall off and the stitching is broken, its eye-catching color is still impressive. Dr. Tyden's task was to restore the book before the exhibition opened. Under a microscope, she saw a black waxy substance attached to the book cover. She tried to brush it off with a soft porcupine quill, but found that thin flakes fell off where the bristles passed. This may not seem strange to others, but Dr. Tyden was very surprised. She began to suspect that the book cover was colored with pigment instead of dye. Pigments and dyes are both colorants, but dyes are usually soluble, while pigments are less soluble and are suspended in the liquid in the form of particles. If the book cover is colored with pigments, it is not difficult to explain why the coating is not very cohesive, and even the lightest touch from a quill pen is enough to remove a hint of color. But when it comes to pigments, especially green pigments, Dr. Taiden can't help but think that the book was published in the 19th century, when some bright and toxic green pigments were the most popular. This made her start to worry that when she encountered the old green book more than 100 years later, would she still be poisoned by the old "fashion trend"? Part 1 Green is so healthy, how can it be poisonous? Green is probably the most abundant color in nature. However, it is not easy to turn this ubiquitous green into a pigment or dye for human use. The green extracted from plants is often unstable. It is green and lush at first, but soon turns into a dull brown. It is difficult to collect a stable green color from plant ingredients, so humans will inevitably turn their attention to minerals. For example, the ancient Egyptians mined malachite, ground it and used it as a green pigment in paintings on the walls of some pharaohs' tombs, dating back to the Fourth Dynasty (2613-2494 BC). In the Renaissance, many artists still used malachite pigments to paint. The main component of malachite is basic copper carbonate, which can retain its original color for a long time in oil paintings and tempera paintings. Raphael's Sistine Madonna, using malachite pigment (Image source: Wikimedia Commons) Since malachite, many copper-containing pigments have left their own green at different times in history. However, most of them may not be as famous as Scheele's Green, which was born in 1775. In 1775, a chemist named Karl Scheele slowly added arsenic trioxide (commonly known as arsenic) to a heated sodium carbonate solution to obtain sodium arsenite, and then poured the sodium arsenite solution into a copper sulfate solution to obtain copper arsenite precipitate. This is Scheele's green, which is bright and not easy to fade, and has a low production cost. After it was launched on the market, it quickly became a star product, leaving other green pigments in the cold. The painting "Woman Embroidering" uses Scheele Green (Photo credit: Wikimedia Commons) This pigment is not only active on the canvas of artists, but also begins to enter every corner of human life. Wallpaper at home, clothes on the body, and children's toys have all become objects of coloring with Scheele's green. Sometimes it is also used as a food coloring and added to candies. Although arsenic trioxide was used as a murder poison in the Middle Ages before that, ordinary people in the 19th century did not have a widespread understanding of the toxicity of arsenic compounds. When people discovered that Scheele's green would turn black when it came into contact with sulfides, they even wanted to create a more durable green pigment based on this. In 1814, two German chemists improved Scheele's formula and used arsenic trioxide and copper acetate to obtain copper acetate arsenite precipitation, also known as "emerald green." After the birth of this bright new pigment, it followed the path of Scheele's green, was favored by many painters, and invaded the lives of ordinary people in a big way. Later, it also had a more resounding name-"Paris Green". Left: Wallpaper with Paris green; right: Van Gogh's self-portrait with Paris green (Image source: Wikimedia Commons) Scheele Green and Paris Green were both color trends during the Victorian era. As these arsenic pigments became popular, so too did the number of poisonings associated with them. The people who came into contact with these pigments the most on a daily basis were probably the workers who were responsible for coloring the goods. In 1861, a 19-year-old girl died. She had been coloring artificial flowers with arsenic green powder in a London factory. At the end of her life, her nails and the whites of her eyes were green. An autopsy found arsenic in her stomach, liver and lungs. When arsenic-rich products leave the factory, consumers may become the next victims of arsenic. In London in 1862, in a family named Turner, parents lost three children in succession, and a surviving daughter also contracted a serious illness. Originally, the doctor believed that the children had diphtheria based on the symptoms, a respiratory infectious disease that was very common at the time. But strangely, other people who often came into contact with those children did not contract the same disease. Moreover, the Turner family and the community where they lived had good drainage and ventilation, and no serious health risks were found. Finally, the doctor finally suspected the green wallpaper in the patient's home. Soon after, the seriously ill child also died. After testing her tissue samples, chemists believed that arsenic poisoning might be the real cause of death. Since then, many similar incidents have gradually made people believe that those arsenic green pigments are harmful. Even if people do not deliberately touch them, the particles that fall off will float into the air. If they are inhaled in large quantities by humans, they may cause physical discomfort, such as dizziness or diarrhea. Moreover, children and the elderly may be more vulnerable than healthy adults if they are exposed to arsenic in an environment for a long time. Later, Scheele Green and Paris Green were gradually abandoned. Today, it is difficult to find these two pigments in anyone's green wallpaper. However, some items that have survived from the Victorian era may still retain the color or toxicity of that time. For example, the book mentioned at the beginning... Part 2 "Poison Book Project" In fact, Dr. Melissa Tyden was suspicious of a 19th-century book not only because she easily scraped green scraps off the book jacket, but also because she remembered that she had seen a Victorian green wallpaper pattern in a recent book, which was very similar to the color of the book jacket she was restoring. Dr. Tyden boldly guessed that the pigment used in the book cover was Paris green. To confirm her suspicion, she sought help from Rosie Grayburn, a researcher who also worked at the Winterthur Museum. Grayburn first used X-ray fluorescence spectrometry (XRF) to analyze the elements in the green book cover and found that copper and arsenic were present in high levels. XRF analysis of books from the 19th century (Image credit: Winterthur Museum) However, XRF can only determine the elemental composition of the sample. As for what kind of molecules those elements form together, further determination is needed. Grayburn uses Raman spectroscopy to study molecular structure. When the laser hits each molecule, a unique scattering spectrum is obtained, which is like a unique "fingerprint" that distinguishes it from other molecules. This method allows scientists to confirm that the pigment on the surface of the book is copper acetate arsenite - Paris green. Although his idea was confirmed, Dr. Taiden was still shocked: "I know that there were arsenic pigments in wallpaper in the past, and I also know that arsenic pigments were used in illustrations in some books, but I didn't expect this toxic substance to cover the cover and you would touch it when you hold the book to read." Not only that, Tyden and Grayburn also found that the book cover contained 1.42 mg of arsenic per square centimeter. If left untreated, the lethal dose of arsenic poisoning for adults is 100 mg. Dr. Tyden believes that he only did some simple treatment on the book, which may not cause major harm, but if it is a library staff, there are more opportunities to come into contact with arsenic pigments, and they will face greater risks. Long-term exposure to inorganic arsenic may cause many health problems, such as skin damage, peripheral neuropathy, etc. Some cardiovascular diseases, respiratory diseases, liver diseases, and even cancers in some organs are also related to inorganic arsenic. Therefore, the two researchers decided to launch the "Poison Book Project" in the hope of finding more books that may be harmful to the human body from old books in the 19th century and cataloging them. In the Winterthur Library alone, they detected 9 "poisonous" books, 4 of which were still circulated between different libraries. In the Library Company of Philadelphia, the oldest library in the United States, they found 28 old books containing Paris green. Books with Paris green on their covers (Image credit: Winterthur Museum) So far, Tyden and Grayburn have found 92 books with Paris green covers. They also distribute "colorimetric" bookmarks to help people identify which colors are more like 19th-century Paris green. Whenever a suspected object is found, the researchers still use XRF to detect elements as they did at the beginning, and if suspicious elements are found, they use Raman spectroscopy to further confirm. Of course, identifying poisonous books is only one step in the plan. What is more important is to reduce the harm of those books to people. Grayburn said that if arsenic penetrates the skin of the hands, it may be dangerous, and it is especially dangerous if it is eaten or enters the body through the respiratory tract. The "Poisonous Book Project" lists some precautions for people who need to come into contact with poisonous books: Before operation, wear nitrile gloves and N95 masks. During operation, avoid eating, drinking and smoking, and do not let the substances on your hands touch your face; wash your hands after operation. In addition, do not operate on soft surfaces (such as sofas), and place the poisonous books on hard surfaces such as tables. After the operation, wipe the surface where the books were placed with a damp cloth to remove the remaining toxic particles. Photos by: Melissa Tedone & Rosie Grayburn Of course, a more fortunate and convenient situation is that you will never come across these poisonous books. Although most libraries with collections from the mid-19th century will have a few bright green books, it may be difficult for ordinary people to encounter them. In addition, not every green book from the 19th century was colored with arsenic pigments. Dr. Taiden also said, "I just happened to come across such a book." References: Original paper: https://www.tandfonline.com/doi/abs/10.1080/01971360.2022.2031457 https://www.iiconservation.org/content/poison-book-project https://www.nationalgeographic.com/science/article/these-green-books-are-literally-poisonoushttp://wiki.winterthur.org/wiki/Poison_Book_Project https://www.finebooksmagazine.com/issue/conservators-find-arsenic-old-books Source: Global Science This article has been authorized for reprinting. If you need to reprint, please contact the original author The article only represents the author's views and does not represent the position of China Science Expo |
<<: Shock? Panic? Has the Big Bang theory been overturned by the Webb telescope?
>>: Can mosquitoes be completely eliminated?
Recommend
YouTube reveals: 5-second ads earn more than 120-second ads
Last weekend, I opened an app and was about to wa...
Don’t just look at the down content! The new national standard has been implemented. When buying down jackets this year, pay attention to this →
As the weather gets colder, down jackets have bee...
Does Starbucks have "medium cup discrimination"? No, this is their marketing!
A while ago, it was the Christmas green cup that ...
The Complete Collection of Chen Qingyun's Novels: How to optimize a new website to get better ranking for keywords?
For new websites, keyword ranking usually takes 3...
World Economic Forum: The impact of artificial intelligence on Education 4.0
If deployed properly, AI can help find solutions ...
Behind Liu Jun’s resignation: Are contradictions in Lenovo’s internal development strategy beginning to emerge?
Recently, Lenovo Group announced a personnel adju...
"I want you" - recruitment copywriting of the thousand-year-old ancient Zen temple, read more than 1,000,000 times, received more than 4,000 resumes
My Buddha is looking for wise men! Recently, Dong...
How do you evaluate Haidilao’s slogan?
Anyone who works in advertising knows that a good...
What 4K lacks is not standards but the maturity of the ecosystem
Recently, the National Radio and Television Produ...
How do the popular blue Vs on Douyin operate content?
Today, Tik Tok has become a battlefield that cann...
The principles and applications of iOS compilation process
Preface Programming languages can generally be ...
5 steps to plan an event promotion!
Whether you are doing user operations, new media ...
The chicken mushroom, known as the "king of mushrooms", is unforgettable to Xu Xiake and Li Shizhen!
Termitomyces albuminosus is a fungus that lives i...
Today is Grain in Ear丨Midsummer begins and the water chestnut song begins everywhere
"Sometimes I see sparse stars falling on the...
How does Baidu bidding charge? Is there any contact information?
Baidu’s paid promotion is charged based on clicks...