How can two drops of water and a piece of paper generate electricity for one hour?
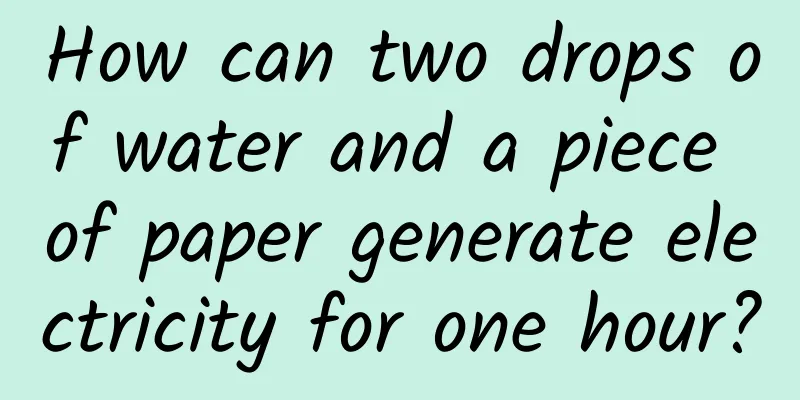
|
In the past few decades, due to the accelerated technological changes, shorter product lifespans, and excessive use of electronic products by humans, e-waste has become the fastest growing waste stream in the world. Data from the United Nations University shows that approximately 53.6 million tons of electronic products were discarded worldwide in 2019. It is estimated that by 2030, this figure will reach 74.7 million tons. If this trend cannot be reversed, it will even reach 110 million tons by 2050. (Source: Pixabay) Using more environmentally friendly materials and improving resource recycling rates are important methods to solve the problem of the proliferation of electronic waste, and biodegradable batteries are a hot topic in current battery research. Now, a research team from the Swiss Federal Laboratories for Materials Science and Technology (Empa) has made a new breakthrough in biodegradable batteries - in a proof-of-concept study, they proposed a disposable paper battery that can be activated by water. Figure | Two-unit paper battery is driving an alarm clock with LED. (Source: Research team) It is reported that with just two drops of water, this new type of battery can continuously power an alarm clock with LED for 1 hour. After 1 hour, add two more drops of water and it can continue to be used. It can also be made into any shape and size, and its potential application scenarios are very broad. The research paper, titled “Water activated disposable paper battery”, has been published in the scientific journal Scientific Reports. Dr. Gustav Nyström from the Swiss Federal Laboratories for Materials Science and Technology is the corresponding author of the paper. The research team said that this paper battery can be used to power a variety of low-power, disposable electronic devices (such as smart tags for tracking items, environmental sensors and medical diagnostic equipment) and minimize their environmental impact. How can paper batteries generate electricity? In recent years, scientists have made important progress in green power technologies such as biodegradable photovoltaic devices, energy harvesters and supercapacitors. However, research on biodegradable disposable batteries, which are highly complementary, provide higher energy density and more stable operation, is still very limited. Current battery research is focused on improving battery performance, which is to continuously develop higher energy and power density, faster charging rates and better operating stability, mainly through the development of new materials that meet the requirements of lithium-ion batteries that currently dominate the market. However, as people become more aware of the hazards of electronic waste and disposable electronics emerge for applications such as environmental sensing and food monitoring, the market demand for environmentally friendly batteries is growing. Despite some promising progress, additive manufacturing of biodegradable batteries remains an important scientific challenge. In the past decade, cellulose, which has existed in the form of paper for thousands of years, has been widely used in biomedical diagnostics, information display and energy storage. However, some of cellulose's unique properties, such as its inherent biodegradability and hygroscopicity, have not been well utilized. In this research work, the paper battery proposed by Nyström consists of at least one battery cell with a size of 1 square centimeter, which contains 3 types of inks printed on a rectangular paper tape. Sodium chloride salt is distributed on the paper tape, and the shorter end is impregnated with wax. One ink contains graphite flakes printed on one side of the paper, which serves as the positive electrode (cathode) of the battery pack. The back of the paper is printed with an ink containing zinc powder, which serves as the negative electrode (anode). In addition, on both sides of the paper, on top of these two inks, are printed inks containing graphite flakes and carbon black, which connect the positive and negative terminals of the battery pack to the two wires that are located at the wax end. These inks are ideal for additive manufacturing technologies such as 3D printing. So, how does this paper battery generate electricity? According to the paper, with just a little water, the salt on the paper will dissolve, releasing charged ions. These ions diffuse on the paper and activate the battery pack, allowing the zinc in the negative electrode ink of the battery pack to release electrons. The wires connected to the electronic device can complete the circuit, allowing the electrons to pass through the graphite and carbon black-containing ink, wires and equipment, and transfer from the negative electrode to the positive electrode (graphite-containing ink), where they are transferred to oxygen in the surrounding air. Electric current is generated in these reactions. Figure|Design of paper battery. (Source: the paper) To demonstrate the battery's ability to drive low-power electronics, the team combined two cells into a battery pack to power an alarm clock with a liquid crystal display. Analysis of the performance of a single-cell battery pack showed that with the addition of two drops of water, the pack activated within 20 seconds and reached a stable 1.2 volts when not connected to a power-consuming device, compared to the 1.5 volts of a standard AA alkaline battery. After one hour, the performance of the single-cell battery pack rapidly degraded due to the drying of the paper. However, if two drops of water were added, it could maintain a stable operating voltage of 0.5 volts for more than an hour. Figure | Characterizing the discharge capacity of disposable paper batteries by measuring the operating voltage at a constant current (100 μA). (Source: This paper) The research team said the biodegradability of paper and zinc allows the battery to minimize the environmental impact of disposable, low-power electronics. Further research is needed As mentioned above, although this paper battery can power an alarm clock, its power density is still limited compared to other types of batteries. In this regard, the research team believes that this paper battery can be used in low-power electronics and the Internet of Things ecosystem. The sustainability of this battery can be further improved by minimizing the amount of zinc used in the ink, so that the current generated by the battery can be precisely controlled. In future work, they will investigate how to use green catalysts to increase the rate of the oxygen reduction reaction, use organic anode materials as an alternative to zinc, and evaluate the environmental impact over the battery's life cycle. "Our work advances the field of disposable electronics and proposes a battery technology that balances environmental impact and performance," the research team said. Reference Links: https://www.nature.com/articles/s41598-022-15900-5 https://ewastemonitor.info/wp-content/uploads/2022/06/Global-TBM_webversion_june_2_pages.pdf |
<<: There are so many colors of sunglasses, how do you choose?
>>: Trivia about how the ancients repelled mosquitoes
Recommend
Behind the super drama "The Advisors Alliance", we get a new era of endogenous advertising
Video marketing has moved from the hard advertisi...
Technology News丨The US hypersonic missile failed three times in a row; Sanxingdui Ruins and Jinsha Ruins jointly applied for World Heritage
【Today’s cover】 In winter, tens of thousands of w...
Will surgery under general anesthesia make people stupid? Is it true that there are many side effects?
Before the advent of anesthesia, surgeons had to ...
Mistakenly thought it was a cold, and almost lost my life! Don’t ignore these situations...
Have you ever had this experience when you had a ...
Four user growth strategies for online education institutions!
How to achieve growth in the field of online educ...
How effective is my country's COVID-19 vaccine? Can elderly people with chronic diseases be vaccinated?
Source: China Government Network The Joint Preven...
How do SEM bidders respond to company interview questions? A collection of interview secrets!
I believe that every SEMer works hard to optimize...
JD.com’s fresh food category operation case analysis: How to build a store?
Sometimes, a store clearly has significant advant...
National Nuclear Emergency Publicity Week丨Nuclear? Nuclear medicine? Learn everything in one article!
In the hospital, when many patients receive the e...
The difficulty is comparable to the docking of a space capsule! For the first time in Olympic history, a robot relayed the Winter Olympics torch underwater
◎ Science and Technology Daily reporter He Liang,...
ACEA: European Electric Vehicle Charging Infrastructure Master Plan (72 pages)
EU-27 Electric Vehicle Charging Infrastructure Ma...
We may have got the tongue's taste map wrong
Eating is human instinct. Especially for foodies ...
vivo Global Mall: Universal pickup code design for e-commerce platforms
Author|vivo official website mall development tea...
The dragon raising its head is a celestial phenomenon. Do you know its relationship with the Chinese totem?
Dragon Raising its Head refers to the phenomenon ...
When will the Beijing epidemic end in 2022? How long will it take to lift the epidemic and remove the star? Attached is the latest news on the epidemic
This round of local epidemic in Beijing has not y...


![[Girls' Emotions] Love psychology to save singles, find a good man to love you](/upload/images/67cc26ab00c4f.webp)